Nordson MEDICAL provides elegant and robust design and development solutions of finished medical devices.
Product Development Process (PDP Services™)
We recently established a new best-in-class Product Development Process, PDP Services™, that will help customers manage their business goals appropriately at the highest quality. Key aspects of PDP Services™ include:
- Alignment with FDA and ISO Regulations
- Best-in-Class Standardization
- Concurrent Engineering
Alignment with FDA Regulations
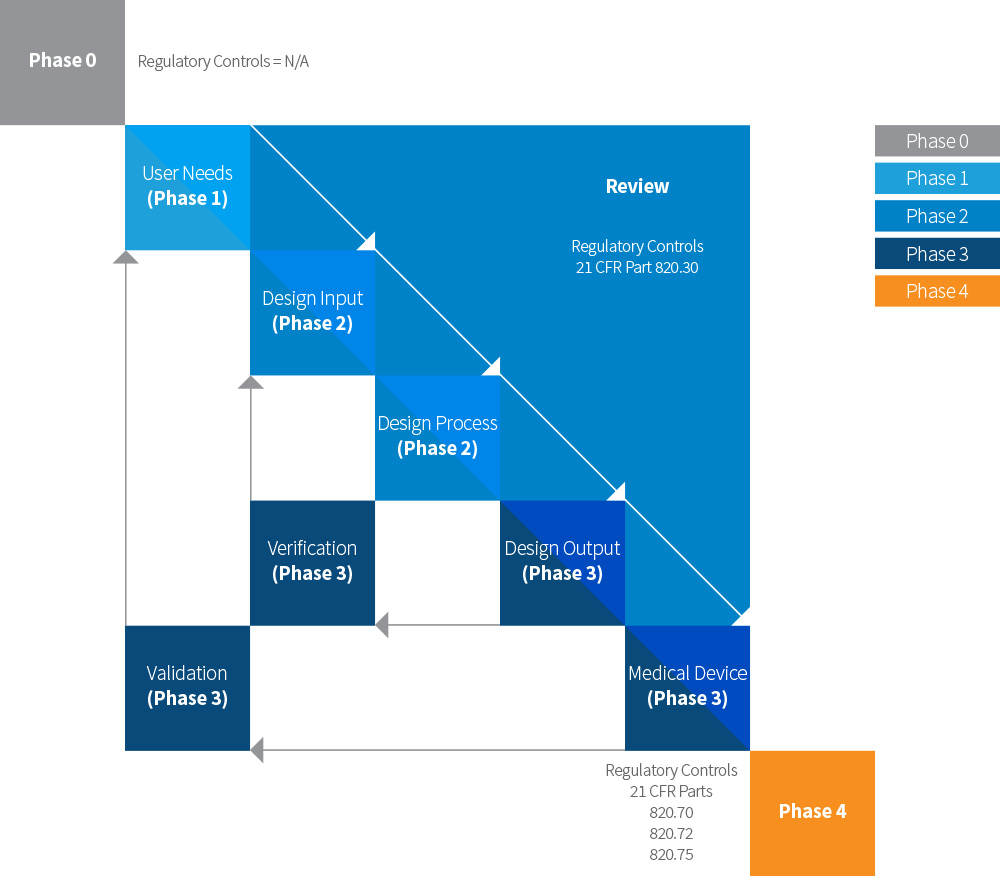
Based on the FDA's waterfall diagram, we aligned our Product Development Process, practices, and procedure requirements.
Best-in-Class Standardization
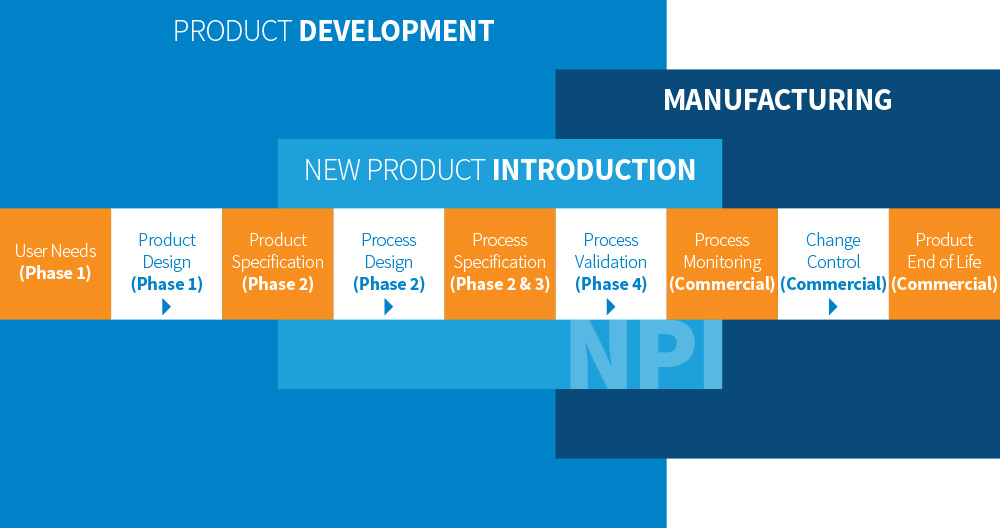
Nordson MEDICAL provides a vertically-integrated offering that includes design, development, and manufacturing of finished devices, from concept to launch. PDP Services™ is aligned to:
- Help our customers achieve their business goals
- Ease cross-site communication
- Ease project transitions and transfers between sites
Concurrent Engineering
The FDA Design Control Guidance for Medical Device Manufacturers explains the benefits of concurrent engineering, which ensures the medical device design and process are developed in parallel. This could result in shorter development time, decreased manufacturing costs, and overall improved quality, which will allow our customers to get to market faster and more efficiently.
Program Management
We take a structured and disciplined approach to project management that allows us to manage medical device programs of any scale and scope. The phase deliverables in PDP Services™ were designed to meet all regulatory requirements. We are fully transparent in timeline and budget tracking as your project progresses.
3R (Review. Research. Recommend.) Design Review Service
Our 3R Design Review Service can reduce technical risk by forging a path for optimizing medical device designs at any stage. Our team can conduct a thorough review and recommend opportunities to refine your product design, improve your manufacturing process, address yield issues, and reduce costs. The result is a comprehensive summary report with detailed, pragmatic recommendations to reduce risk and improve design reliability.
Why 3R?
- Need your prototype design to be more scalable and cost effective to manufacture?
- Challenged with low cost-of-goods-sold (COGS) targets?
- Concerned about reliability and scalability in production?
- Need to improve product performance?
- Moving a development project to a new supplier?