Speed to market has become a key factor of success in the biopharmaceutical industry, yet it can be difficult to achieve without compromising product quality. In an attempt to avoid sacrificing either competitive advantage, companies continuously seek out products that are able to provide maximum efficiency while maintaining first-rate quality. These requirements were recently designed into a new bag port line manufactured by Nordson MEDICAL.
Two aspects new to the industry and unique to the bag port series are flow alignment ribs and parabolic entrance geometry. These features, respectively, promote a uniform flow by guiding the media into a consistent, rotational path while reducing the flow separation from the wall. In order to validate this performance, extensive testing and analysis was carried out and then used in comparing the bag ports to other commercially available products.
Computational fluid dynamics (CFD) analyses were also performed. In these studies, the Nordson MEDICAL Bag Ports saw a 30.86% increase in volumetric flow rate when compared to other commercially available products. This can be attributed to the bag port's curved entrance which promotes a consistent velocity profile being maintained throughout the flow path and a near elimination of flow separation. The result is a maximized use of space, allowing the largest amount of media to flow through the given inner diameter.
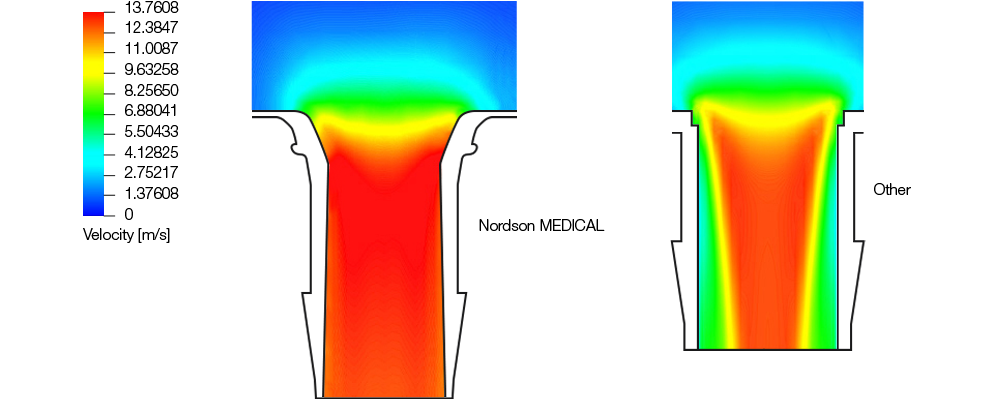
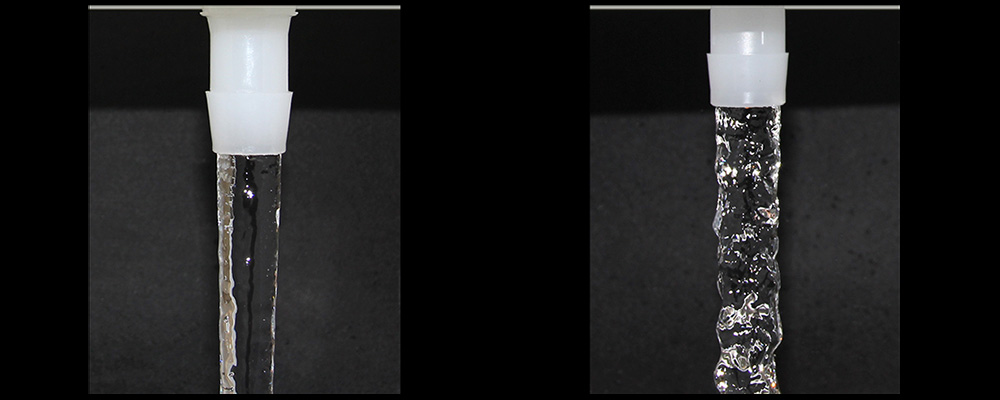
Flow characteristics were observed in additional testing. While the square-shouldered lead-in bag port generated an erratic turbulent flow, the parabolic lead-in produced a straight streamline flow. Incorporating the alignment ribs led to the eventual streamline rotating flow, providing the media with a more consistent and stable path.
Validation Test Results
Validation testing was performed on the two available bag port designs – barbed and ferrule. The features assessed included, but were not limited to:
- Aged Environmental Stress-Cracking Resistance (ESCR)
- Chemical Compatibility
- Post-Alcohol Stress During Extended Use
- Barb Fatigue
- Suction Relief
- Hydrostatic Pressure
- Side Load
- Impact
- Gamma Sterilization
- X-Ray Sterilization
- Leak
- Pressure
ESCR with Alcohol Testing in particular was a key milestone to overcome in proving these components. Many polyethylene bag port components may experience cracking when exposed to alcohols and stress. This experimental procedure helped validate that Nordson MEDICAL materials have appropriate ESCR, ensuring that cracking will not occur during the designed life of the bag ports.
Nordson MEDICAL's positive results in these tests are coupled with other critical features in biopharm applications such as an animal-free resin, USP Class VI verification, and guaranteed heat sealing and conformance with polyethylene bags. The Value Pharma™ resin used in these ports is unique in the market due to the combination of these qualifications.